Pipeline
PIPELINE
Cellular Immunotherapy in development:
Watch the video
CIM201: Adoptive NK cell therapy (Acute Myeloid Leukemia)
The lead product CiMaas is developing is a NK cell product for AML. In addition, CiMaas is generating procedures to optimize the proliferation and potency of NK cells. Currently, we are capable of expanding NK cells 5.000-10.000 fold in 12 days with our proprietary technology using in house generated feeder cells. The cytotoxicity of these cells is very high, even after a freeze/thaw cycle. This expansion technology functions as a platform for treatment of many types of cancer. CiMaas aims to start a phase I/IIa clinical trial using these powerful expanded NK cells for AML in Q4 2024.
CIM401: Adoptive CAR-NK cell therapy (Solid tumors)
To further improve NK cell activity, CiMaas develops Chimeric Antigen Receptor (CAR)-NK cells for solid tumors. Toxicity of CAR-NK cells is expected to be lower than CAR-T cells. The CARs are directed against tumor-specific underglycosylated proteins. The first focus here is on breast cancer, but can be applied in many solid tumors.
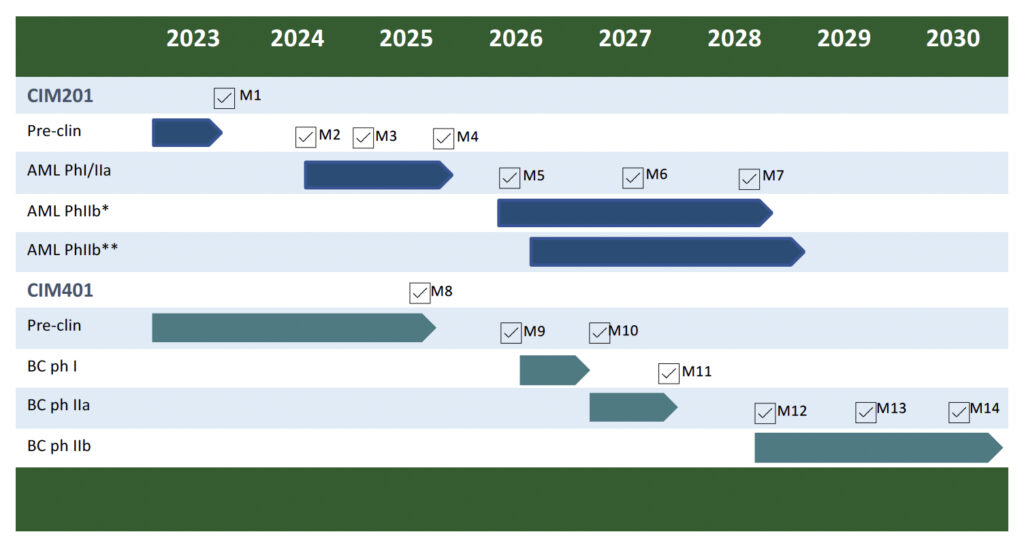
M1 – M14 are anticipated milestones.
CIM601: Dendritic cells vaccine (Lung Cancer)
The second product platform for CiMaas is a therapeutic dendritic cell vaccine using a proprietary maturation technology. For this development CiMaas is seeking collaboration and has out licensing opportunities.
