Adoptive NK cell therapy
ADOPTIVE NK CELL THERAPY
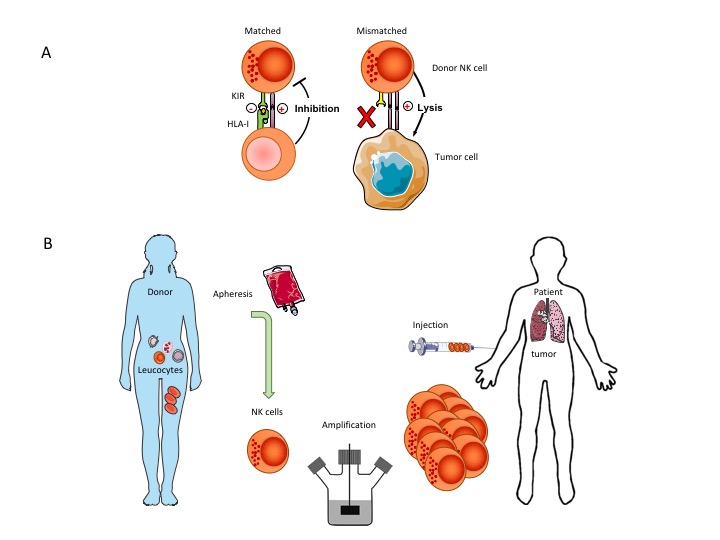
NK cells are the gatekeepers of the innate immune system. They are able to efficiently kill cells in case activating ligands are present and signals for inhibitory receptors are absent.
After “danger” cell recognition, granules from natural killer (NK) cells release perforins and granzymes that induce apoptosis (programmed cell death) in target cells. This usually occurs against virus infected cells or tumor cells with diminished expression of class I MHC. However, normal unaltered cells remain unaffected. In addition, NK cells can bind antibody bound to a target cell and subsequently kill the target cell through a mechanism called Antibody Dependent Cellular Cytotoxicity (ADCC).
NK mediated cell killing is a powerful mechanism to remove cancer cells leaving healthy cells intact. Haploidentical NK cells (i.e. from a family member) have a partial mismatch in killer inhibitory receptors and are thus more easily activated when stress ligands are recognized (which are very highly expressed on tumor cells). CiMaas scientists have a long-standing research line on the use of donor NK cells in cancer. Pre-clinical and clinical research has demonstrated that the most optimal response is generated through an allogeneic Killer-cell Immunoglobulin-like Receptor (KIR) mismatched adoptive transfer using selected donor NK cells. Purposely mismatching KIRs by proper selection of donors circumvents the inhibitory effect of self-recognizing KIRs after infusion.
CiMaas developed in an International collaboration an optimized NK cell expansion technology capable of producing sufficient NK cells to treat a patient. Until now, generating such large numbers of NK cells has been a major bottleneck in the development of adoptive NK cell therapies. CiMaas has developed a proprietary, fully closed manufacturing procedure for primary NK cell expansion and has generated preclinical data of the activity of these cells against various malignant cell types. To our knowledge, we are at present the only company in Europe that can expand primary NK cells from a low number of white blood cells (present in between 150-200 ml blood) to the number of NK cells needed to treat a patient in the shortest time possible with the right phenotype and function. CiMaas’ donor derived, patient specific NK cells can become available within 2 weeks after donation allowing a rapid start of the treatment. The methodology furthermore has the advantage that no other starting product is needed like cord-blood cells from a cord blood bank or induced pluripotent stem cells, or the use of immortalized and irradiated NK cell lines.
At the same time, cells can be obtained from an in house established general blood-cell bank for a large group of genotyped unrelated donors to match the patients, thus creating an off-the-shelf product.
The NK cells have been tested in vitro and compared in killing capacity to freshly isolated, IL2 stimulated NK cells. The data show that after thawing the expanded NK cells can dramatically kill all tested Acute Myeloid Leukemic cell lines in a short period of 4 hours far better than fresh NK cells.
Furthermore, the combination of genetically mismatched expanded NK cells with an antibody can kill multiple myeloma cells that are very difficult the kill by matched NK cells with out antibody. This way if killing is facilitated by the high CD16 expression on expanded NK cells.
CAR-NK cells
Furthermore, CiMaas has technology under further development to improve the function of NK cells – focussing on CAR-NK cells to even treat more treatment resistant diseases; especially focusing on the most frequent deadly type of tumors, like breast cancer in women. Hence, CiMaas’ NK cell platform allows for a pipeline of multiple generations of NK cell products, including unmanipulated cells for hematological cancers and enhanced NK cells for solid tumors. The CARs are directed against tumor-specific underglycosylated proteins.
